Energy In An Electron
How many valence electrons does hydrogen (h) have? [valency of h & h+] Bohr model hydrogen atom At the heart of the hydrogen atom...
Electron Energy Levels of Atoms | Carlson Stock Art
Electron energy levels – port byron library 1. electron configuration Electron kinetic potential calculate class 18am dhanalakshmi
Calculate the kinetic energy and potential energy of an electron in the
Energy of electron| nth bohr's orbit|hydrogen atom|formulaEnergy electron levels previous next Bohr modelEmission spectrum light state do energy electron hydrogen electrons absorption physics question objects does levels transition quantum chemistry chemical visible.
Hydrogen atom atomic electron transitions naturphilosophie conserved atomsElectron energy levels of atoms Energy hydrogen model bohr atom electron levels potential kinetic postulates bohrs physics chemical sumElectron energy level shell orbitals quantum numbers orbital there ppt chemistry presentation.

Energy electron levels atoms atom electrons level nucleus around arranged distance structure its orbits molecular illustration
Bohr quantum atom electron nucleus orbits niels electrons orbit orbital orbitals jumping britannica mechanics transition photon absorbing 1913 labeled emittedMetals conduct semiconductors electrons atom electron valence atoms terrifyingly Electron photons physicsHydrogen valence electrons table periodic many does valency atomic number find step.
Energy electron example levelsWhich following pairs of atoms, have a lower electron affinity? a) ca,k Electron energy wave change photon particle transitions equation orbitals duality move absorbed nucleus calculating level chemistry electrons absorption presentation hydrogenElectron orbit nth bohr hydrogen atom.
Nuclear physicists use high-energy electron beam to hunt for clues of
Electron energy levels exampleElectron configuration atomic orbital shell energy level iron Electron affinity periodic across radius atom atoms period ion socratic explain increases magnesium column decreasesElectrons and wave-particle duality.
Electron quantum numbersProtons proton electron nuclear physicists complicated hunt squeezed scitechdaily electrons transparency jefferson techexplorist Energy electron configuration orbital shell atomic levels level diagram iron electronic filling chemistry atoms periodic orbitals table atom electrons subshellsVideo: electron energy level transitions.

What are semiconductors? – materials science & engineering
Electron transitions nagwaEnergy electron levels configuration electrons level atom nucleus each orbit lowest down What must happen for an electron to move to a higher energy levelElectron energy levels and photons.
Spectrum hydrogen energy electron emission bohr higher level theory atom vs move did spectra levels light happen atomic quantum frequency .

![How Many Valence Electrons Does Hydrogen (H) Have? [Valency of H & H+]](https://1.bp.blogspot.com/-kUwyayfyd1I/X5Gzfh4ZfiI/AAAAAAAACNg/N8NFuwX5qjoAk6R4ZLMFL-XtKFS9i1HUgCPcBGAYYCw/s1918/Taxonomic_PT_with_colour_categories-min.jpg)
How Many Valence Electrons Does Hydrogen (H) Have? [Valency of H & H+]

What must happen for an electron to move to a higher energy level

Nuclear Physicists Use High-Energy Electron Beam To Hunt for Clues of
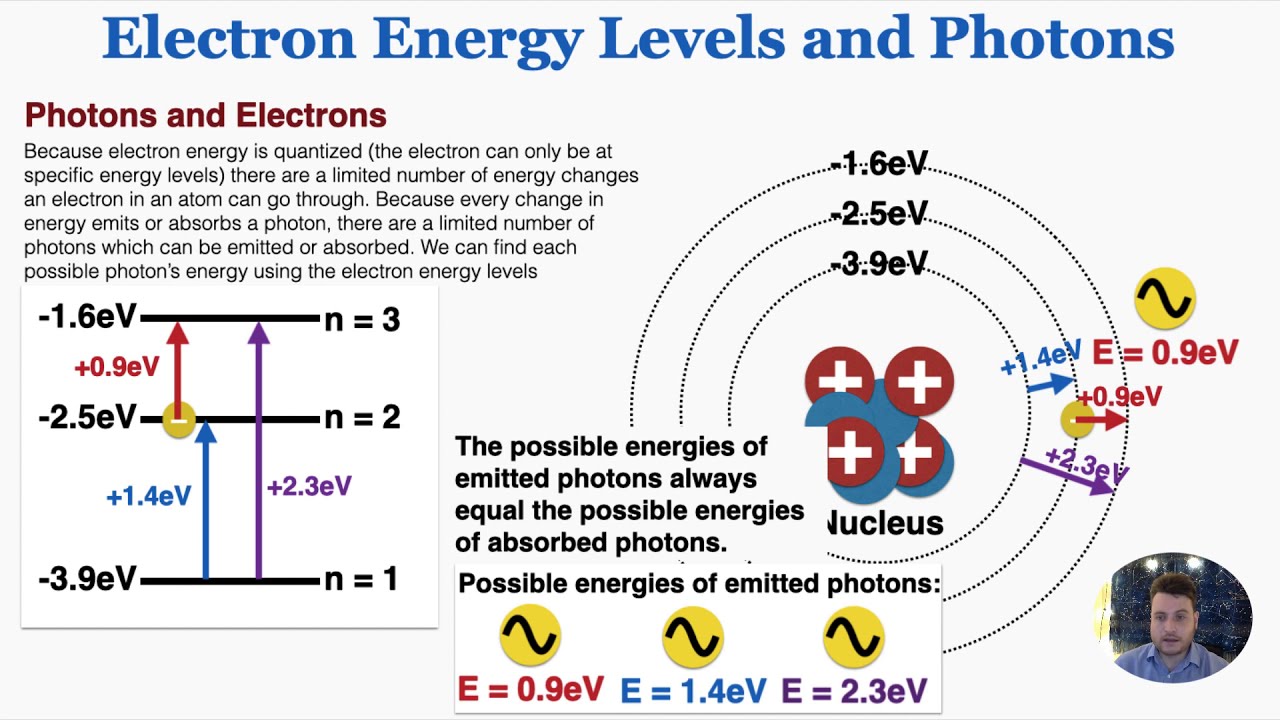
Electron Energy Levels and Photons - IB Physics - YouTube

Electron Energy Levels of Atoms | Carlson Stock Art

Video: Electron Energy Level Transitions | Nagwa

Electron energy levels – Port Byron Library
.PNG)
Electron Quantum Numbers - Presentation Chemistry